Unique Device Identification Technologies Automate Medical ID and Barcode Reading
Contributed By DigiKey's North American Editors
2023-10-26
In 2013, the U.S. Food and Drug Administration (FDA) implemented the Unique Device Identification System or UDI rule. The rule aimed to improve patient safety by providing a consistent method of medical device tracking and identification using modern documentation at manufacturing, distribution, and use points. Much like the Medical Device Regulations Requirements in Europe or similar regulations in other countries, the U.S. UDI rule boosts reporting accuracy and facilitates analysis in the event of a recall or adverse event.
As of September 24, 2023, the FDA will fully enforce the National Health Related Item Code and Drug Code Numbers on Device Labels and Packages. Any medical products labeled on or after that date must fully comply with UDI mandates. This mandate will impact the following:
- Class 3 life-critical products such as pacemakers and implanted prosthetics
- Class 2 moderately critical products such as syringes, catheters, and absorbable sutures
- Class 1 lower-risk products such as dental floss, medical gowns, and oxygen masks
Full enforcement of the mandate means that if a medical device does not have a scannable barcode, it will no longer be considered a valid/usable product, even if there are human-readable labels on it and even if the product is generally assumed to be easily identifiable by most users. This full enforcement will spur comprehensive adoption by the medical industry and medical insurance billing.
 Figure 1: Some handheld direct part mark (DPM) barcode verifiers feature advanced lighting and other software to automatically find symbols and cycle through settings to optimize the reading of the specific DPM mark and material substrate at hand. In fact, the LVS-9585 shown here can verify both DPM parts and printed labels for comprehensive analysis and reporting. (Image source: Omron Automation)
Figure 1: Some handheld direct part mark (DPM) barcode verifiers feature advanced lighting and other software to automatically find symbols and cycle through settings to optimize the reading of the specific DPM mark and material substrate at hand. In fact, the LVS-9585 shown here can verify both DPM parts and printed labels for comprehensive analysis and reporting. (Image source: Omron Automation)
The contracting entity (brand bearer) is responsible
In the U.S., the IP owner and user-facing brand of every medical product is responsible for the accuracy and quality of UDI codes. This is especially important as so much of the medical-product market is contract-manufactured and outsourced to other organizations’ facilities. Therefore, it is the responsibility of the contracting organization to ensure its entire supply chain is UDI compliant and producing accurate labels.
Unique device identification technology origins
UDIs are static device identifiers. However, changes to the quantity of items in a package may trigger the need for a new identifier. Issuing agencies dictate how these details are distinguished. In the same way, changing a device’s packaging sterility conditions may also change the device identifier. Changing a device’s destination market (the country where a device will be sold), label language, or CE mark may also necessitate changes to the device identifier.
Prior to the UDI rule, a medical-device manufacturer may have labelled a product with a particular part number. The distributor would change that part number before the healthcare provider or hospital would change it again. With the possibility of every entity changing the part number before reaching the patient, it was nearly impossible to track products, handle recalls, prevent counterfeiting, or accurately order new stock with efficiency.
Related: Implementing Robust Traceability Solutions
Presently, a standardized and persistent identifier, called a UDI, is given to every device to allow all entities more rapid and precise device identification, ultimately reducing medical errors. This UDI is an alphanumeric code containing two key pieces of data:
- A device identifier
- A production identifier
A device identifier is a static label assigned to a given device listing the labeler (typically the device manufacturer) and the specific model number of the device. In contrast, a production identifier contains data that can vary, and much of the data it can contain is optional. These can include lot and batch codes, serial numbers, expiration dates, and manufacturing dates. In short, the optional data could be anything a manufacturer or labeler deems necessary to support device tracking.
Every UDI label must present this information in two forms:
- Human-readable form (plain text)
- Machine-readable form (readable by a barcode or RFID reader)
Wherever a device can satisfy multiple medical applications, the UDI must be directly marked onto the device, not on its packaging. The rule also applies to devices that can be used multiple times.
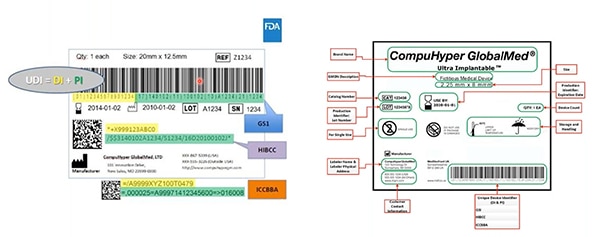 Figure 2: GS1, HIBCC, and ICCBBA — UDI issuing agencies— create UDIs; assign UDIs allowable symbology; define which technologies can interface with UDIs; and specify the required quality of UDI marks. Highlighted in yellow on this sample is the device identifier; highlighted in green are production identifiers. Other elements include human-readable information … and that’s also encoded into the machine-readable barcode. (Image source: FDA)
Figure 2: GS1, HIBCC, and ICCBBA — UDI issuing agencies— create UDIs; assign UDIs allowable symbology; define which technologies can interface with UDIs; and specify the required quality of UDI marks. Highlighted in yellow on this sample is the device identifier; highlighted in green are production identifiers. Other elements include human-readable information … and that’s also encoded into the machine-readable barcode. (Image source: FDA)
All devices must be registered in the global UDI database to allow tracking in the event of a recall and to give the public access to information about a given device. FDA-accredited issuing agencies are authorized to create unique identifiers for device manufacturers to place on their products.
Related white paper: Staying Current on Traceability
UDI scanning technologies and techniques
UDI labels can be verified in several ways along their journey from manufacture to use.
Inline verification is performed by technologies integrated into larger machinery for the quick and accurate processing of vast numbers of product as it is produced. Supported by advanced software, these technologies occasionally take the form of industrial-grade label printers. These printers are capable of their own inline verification to confirm the UDI information is readable to exacting industry standards immediately upon its creation at the point of label production. For example, Omron Automation V275 verifiers are for use with Zebra thermal printers to comply with ISO 15426 and issuing-authority GS1 standards for FDA compliance.
Related: Omron Traceability Solutions
Elsewhere, inline verification takes the form of specialized machine vision flanking conveyors on automated production lines, incorporating barcode reading for extremely fast and accurate verification of UDI labeling on high-mix products on high-speed production lines. Omron Automation MicroHAWK offerings excel in this area with advanced sensors complemented by a miniaturized construction and connectivity options that include Ethernet/IP and PROFINET.
Related: Avoid the Liability of Mislabeling
In contrast, offline UDI verification is the most suitable for the batch sampling of labels to ensure quality. Often employed for sample testing when medical devices leave or arrive at a facility, offline verification can complement online verification systems upstream in the distribution journey.
 Figure 3: UDIs directly marked on products require different verifiers than those used for UDIs printed on affixed labels. (Image source: Omron Automation)
Figure 3: UDIs directly marked on products require different verifiers than those used for UDIs printed on affixed labels. (Image source: Omron Automation)
In fact, all medical-distribution and healthcare operations can benefit from the use of ISO-compliant verifiers. Consider Omron’s LVS 95XX-series offline UDI verification products. These are employed:
- On laser-marking stations and label printers where codes are created
- Where codes are applied to products, which may or may not be separate from the code-creation area
- In quality-control stations where templates, formatting, and other code elements are confirmed
Specifying offline UDI verifier variations
The most suitable offline UDI verifier for a given application depends on several parameters.
Barcode size: Large barcodes are often easier to scan with identifiers having a large field of view defined by lens focal length and sensor size. Consider Omron Automation’s LVS-9510 desktop UDI identifiers. This series of product can read both linear and 2D labels. Five different versions each have a different field of view so designers can select the version to be compatible with the size of the barcode needing to be verified. A stitching feature allows grading of barcodes exceeding the field of view.
In addition, all LVS-9510s can automatically determine the symbology and aperture needed to evaluate the code and identify and highlight trouble spots.
 Figure 4: Barcode verification satisfying ISO standards is made easier with equipment that can verify both linear (1D) and two-dimensional (2D) codes. Some such equipment determines the symbology and aperture needed to evaluate codes and identifies and highlights issues. The LVS-9510 shown here has a stitching feature that allows grading of barcodes larger than the field of view. (Image source: Omron Automation)
Figure 4: Barcode verification satisfying ISO standards is made easier with equipment that can verify both linear (1D) and two-dimensional (2D) codes. Some such equipment determines the symbology and aperture needed to evaluate codes and identifies and highlights issues. The LVS-9510 shown here has a stitching feature that allows grading of barcodes larger than the field of view. (Image source: Omron Automation)
Barcode type: Scanners must read barcode formats as allotted by the issuing bodies known as the HIBCC, ICCBBA, or (most commonly as of 2023) GS1. The GS1 dictates the size, format, and resolution of UPCs, linear barcodes, and 2D data-matrix barcodes.
UDI mark location: Consider the direct part marking (DPM) of medical devices. These markings can be incredibly tiny, especially when they identify surgical instruments and implantable medical devices. To read and verify such DPM UDIs, Omron’s LVS-9580 and LVS-9585 ultra-high-density handheld verifiers feature a specialized lens that can grade myriad DPMs, including those with cell sizes down to 0.002 in. Industrial-grade lenses inside the LVS-9580 and LVS-9585 ensure consistent read accuracy. The most sophisticated are the highly controllable and calibratable lighting technologies inside the scanners. Along with multiple fields of view, the lighting allows immediate optimization of the captured UDI images. This is particularly important as the code standard allows for no after-the-fact corrections or image manipulations.
More on UDI scanner software
For maximum effectiveness, UDI software to support verifier hardware must present diagnostic information in an intuitive format. This software must grade UDI codes according to ISO-defined parameters (most importantly to confirm its readability) and should ideally track worsening issues over a series of UDI scans as well.
Omron includes all necessary software with every one of its UDI-scanning hardware. The software is regularly updated to keep pace with rapidly evolving regulations and new industry legislation.
A major function of Omron scanner software is handling code syntax. In short, every barcode or 2D code must accurately extract all pertinent information including product type, lot number, quantity as applicable, expiration date, shipping information, etc. Resulting data strings must be formatted in a particular way to keep pace with evolving requirements, methodologies, and medical-product destinations.
Regularly-updated software from the scanner manufacture ensures end users stay current even as new codes are released.
Conclusion
The U.S. military once aimed to fully employ a UID product-tracking system like the UDI system of the medical industry. Its purpose was to curtail the massive waste associated with lost, duplicated, and uncounted supplies at military installations including everything from completed Raytheon weaponry to control boards supplied by a small outfit for some specialized application. Today, UIDs see mixed levels of use.
The same will not be the fate of the UDI mandate.
The mantra driving UDI adoption is straightforward: The label is the product. After all, an incorrect label can cause a chain of events that wastes medical staff time and results in the medical device being thrown away. Therefore, UDIs must appear on all layers of packaging such as at the unit, package, bundle, case, and pallet level. UDIs on sterile medical devices inside sterilization seals are especially important, as breaking the seal to verify the device type is unacceptable.
With the FDA requiring full adherence to UDI directives starting September 24, 2023, advanced scanning technologies for verifying medical products will become paramount to automation manufacturers as well as the machine builders and end users they serve.
Such scanners can satisfy extremely specific FDA UDI requirements to confirm labels on a wide array of laboratory, medical, and clinical diagnostic equipment. Offline barcode verifiers Maximize traceability, inline machine vision, and advanced DPM readers.

Disclaimer: The opinions, beliefs, and viewpoints expressed by the various authors and/or forum participants on this website do not necessarily reflect the opinions, beliefs, and viewpoints of DigiKey or official policies of DigiKey.









