Board-Mount DC/DC Converters in Medical Applications
2019-07-23
Medical devices placed on the market in the European Union must meet the essential requirements of directive MDD:93/42/EEC, Article 3 and products placed on the market must meet risk analysis essential requirements set out in Article 3, Annex I, ER1, Annex II, Annex III and Annex VII. The regulations are to ensure device safety and basic performance, specifically, protection of patients and operators against electric shock. Compliance is presumed if a product conforms to ES/EN/IEC 60601-1, currently in its 4th edition.
The need for isolation
If an AC/DC converter is designed into a medical application, the dangers are obvious – if its isolation fails, you could have a lethal voltage on connections to sensors or devices in contact with operators or patients or even to internal organs during surgery. Isolation requirements are a focus, therefore, for the safety standards with levels defined as ‘Measures of Protection’ (MOPs) with more onerous requirements for patient-connect (MOPP) compared with operator protection (MOOP). There must be a minimum of two MOPs overall in each category and there are three further classifications of connections:
Type B: No electrical contact with patient and may be earthed
Type BF: Electrically connected to patient but not directly to heart, floating
Type CF: Electrically connected to the heart of the patient, floating
To meet the standards, the constructional requirements of equipment are also affected by environmental factors such as pollution degree, system voltage, over-voltage category of an AC supply and altitude. Earthing method is also an important factor: whether the equipment is ‘Class II’ earth free or ‘Class I’ with a functional (FE) or protection earth (PE) connection present.
Isolation may also be needed in DC/DC converters
DC/DC converters will often be seen in medical equipment converting one low voltage from an AC/DC converter or battery to another low voltage, perhaps for a processor supply at 3.3 V or sensor power. Functionally, isolation may not be required but is often included to break possible ground loops, to provide a negative rail or perhaps to reduce Electromagnetic Interference (EMI) effects. Sometimes though, even with low input and output voltages, within the definition of ‘safe’ levels, isolation is necessary for patient or operator safety for different reasons.
A DC/DC converter may be part of an overall ‘safety system’
It is possible to procure AC/DC converters fully certified to medical safety standard IEC 60601 (EN 60601 in Europe and ANSI/AAMI ES 60601 in the US) with the necessary 2 x MOOPs or 2 x MOPPs to the highest category of ‘applied part’. These parts are necessarily expensive though with their limited market compared with IT- or industrial-grade parts that are functionally the same but meet EN-60950-1 (or now EN-62368) for safety. The medical standards do however allow levels of isolation to be placed in ‘series’ to achieve higher grades so, for example, an IT-grade AC/DC converter can be followed by an isolated DC/DC converter with the appropriate specification to provide power for just the patient- or operator-accessible parts of the equipment with significant overall cost savings. The IT-grade AC/DC converter may need some modification though: if Class I, it will almost certainly exceed the medical specification for AC mains leakage current through its in-built EMC suppression components – ‘Y’ capacitors between line and neutral and ground. These can be removed however, if EMC is controlled in other ways to meet statutory limits, which have anyway changed in the latest 4th edition of IEC 60601. Medical standards also require both AC live and neutral to be fused, each with a breaking capacity of 1500 A, so using an IT- or industrial- grade converter is not trivial but can be a viable solution. For example, an IT AC/DC converter complying with EN 62368 may have ‘reinforced isolation’, equivalent to 2 x MOOPs or just 1 x MOPP. If it is followed by a DC/DC converter rated at 1 x MOPP, an overall 2 x MOPP is achieved (Figure 1).
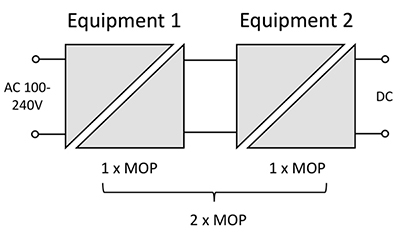 Figure 1: ‘Measures of Protection’ can be added. (Image source: RECOM Power)
Figure 1: ‘Measures of Protection’ can be added. (Image source: RECOM Power)
Fault current from other equipment through the patient must be prevented
Risk analysis of a medical device considers failure of other un-related equipment that might connect to the same operator or patient. At worst, the other equipment may fail and connect high voltage to its external connections with lethal current then flowing through the patient to ground. Patient connections must therefore be isolated from ground to a prescribed level allowing a maximum leakage current at 50/60 Hz. The levels allowed depend on the application but are as low as 10 µA for patient-connect type CF under ‘normal’ conditions (Figure 2).
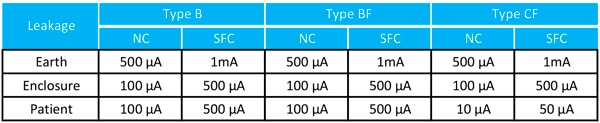 Figure 2: Maximum allowed leakage currents in different medical applications. NC = Normal Condition. (Image source: RECOM Power)
Figure 2: Maximum allowed leakage currents in different medical applications. NC = Normal Condition. (Image source: RECOM Power)
It is very difficult, in even the highest specification AC/DC converters, to guarantee that this maximum level of leakage on a DC output is not exceeded due to distributed leakage capacitances to ground summing. An effective solution though is to power the patient connection from a DC/DC converter which can easily be designed for very low barrier capacitance and hence low leakage current. The specification of the DC/DC isolation depends on what other connections there are to its primary, whether Class I or Class II AC line or battery derived, intentionally grounded or with unspecified connections through, for example, the grounded screens of data interface cables.
In the example of Figure 3, a Class II (plastic cased, earth free) AC/DC with 2 x MOOP isolation rating feeds a DC/DC converter rated at 1 x MOPP. Because the DC/DC converter input is isolated from AC and ground by 2 x MOOPs (240 VAC) with no possibility of other external connections, overall 2 x MOPP is achieved, allowing direct patient connection in a BF or CF application. This is as long as the DC/DC converter has less than 10 µA leakage current at 240 VAC 60 Hz, corresponding to less than 110 pF input-output capacitance.
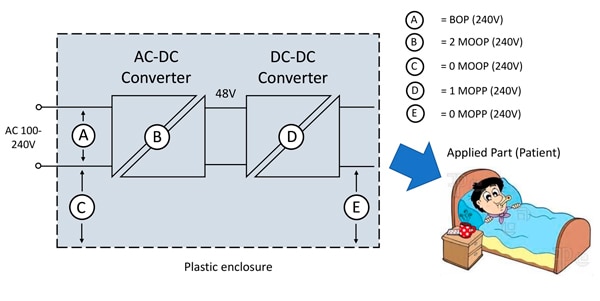 Figure 3: A possible connection scenario requiring a high isolation DC/DC converter, Class II equipment. (Image source: RECOM Power)
Figure 3: A possible connection scenario requiring a high isolation DC/DC converter, Class II equipment. (Image source: RECOM Power)
In the example of Figure 4 however, there are unspecified external signal connections (SIP/SOP) on the input to the DC/DC converter. In this case, to achieve 2 x MOPP, the DC/DC converter must itself be rated for 2 x MOPP, irrespective of the AC/DC converter rating as an external fault could make the SIP/SOP connections live.
Isolation from the DC/DC converter output to the metal casing (E) must also be 1 x MOPP. If the SIP/SOP connection is specified as having 2 x MOOP isolation for the system voltage, and cannot within reason be accidentally substituted, barrier D can reduce to 1 x MOPP. ‘BOP’ in the diagrams means ‘Basic Opposite Polarity’ isolation between AC line and neutral.
 Figure 4: A possible connection scenario requiring a high isolation DC/DC converter, Class I equipment. (Image source: RECOM Power)
Figure 4: A possible connection scenario requiring a high isolation DC/DC converter, Class I equipment. (Image source: RECOM Power)
Other DC/DC converter specifications
Leakage current and isolation in normal and single fault conditions are the main issues for DC/DC converters in medical applications, but there are also other requirements; there are temperature limits for critical components such as the internal barrier transformer and material flammability level must be appropriate. A risk analysis must also be performed and documented, considering all possible hazards such as fire, smoke, burning, and mechanical.
Practicalities of DC/DC converter design for medical applications
It can be very challenging to incorporate the required creepage and clearance distances in miniature DC/DC converters, particularly inside and around the transformer. In the transformer, specialized wire with multiple insulation layers will typically be used. Triple insulated wire (TIW) with three helical, overlapping layers of suitably rated insulation material is often the solution, achieving at least 2 x MOOP rating at 240 VAC. Input-output capacitance of small DC/DC converters can be very low, a few pF, giving low leakage current. Types should be chosen with low EMI emissions though, to avoid the necessity of external input-output suppression capacitors which would allow leakage current and would have to be safety-rated types, often two in series to meet regulations.
Off-the-shelf solutions
The challenges of designing DC/DC converters for medical applications have been addressed by manufacturers such as RECOM [1] with their low-cost REMxE range of modules. Power ratings include 3.5 W, 5 W, and 6 W in DIP24 through-hole packages with SMT parts in the pipe-line. The DC/DC converters have a 2:1 input range with 5 V, 12 V, 24 V and 48 V nominal inputs and a choice of fully-regulated single and dual outputs. All parts have 2 x MOPP rating at 250 VAC with > 8 mm internal/external creepage and clearance and with maximum 1 µA leakage current. An operating temperature range of -40°C to +85°C is standard with no derating for the 3.5 W part. The 5 W and 6 W parts derate from 80°C and 75°C, respectively.
Medical device safety ratings can be achieved with the aid of convenient board-mount isolated DC/DC converters – parts from RECOM meet the required specifications at attractive prices.
References
- RECOM: www.recom-power.com/medical
Disclaimer: The opinions, beliefs, and viewpoints expressed by the various authors and/or forum participants on this website do not necessarily reflect the opinions, beliefs, and viewpoints of DigiKey or official policies of DigiKey.







